Introduction by Mark Hoddle
Have you ever wondered why only a small fraction of introduced species of plants and animals become invasive while others remain well behaved in their new home? This is a puzzling question for invasion biologists and regulators developing plans to manage invasive species. Dr. Norman Ellstrand, a Professor of Genetics in the Department of Botany and Plant Sciences at UC Riverside has studied this issue. In a new contribution to CISR, Norm provides two different ideas for us to think about: (1) some species are “born bad” and they have a natural ability to invade and become pests quickly, while (2) other introduced species that were initially well behaved in their new home for a very long time, perhaps a 100 years or more, under go slow genetic changes as they adapt to their new environment and they evolve to become invasive! You can read about more of these really interesting ideas.
Can Invasiveness Evolve?
Article by Norman Ellstrand
Not all introduced species become successful invaders in their new environments. Scientists have put considerable effort into trying to predict which introduced species are mostly likely to become invasive. For years invasive species scientists sought to identify biological attributes that help exotic species become successful invaders. Until recently, little attention had been given to the possibility that invasiveness might actually evolve after a species colonizes a new area.
Consider these two possibilities: Are invasives “born”, that is, once they get to a new environment, are they biologically pre-adapted to invade? Or are they “made”, that is, do they undergo genetic change after colonization to evolve invasiveness?
Certain characteristics that could promote invasion, like reproductive strategies (e.g., sexual vs asexual reproduction) have been identified, but these are not consistent and reliable predictors for all invaders, suggesting at least some species arrive in a new location ready to invade, that is they are “born” invaders. Also, Charles Darwin’s observation that species from a non-native genus are more likely to be successful invaders than those that are members of a genus already present in the colonized region supports the idea that invaders are born. Certain specific cases of invasives fit this model well. For example, the fact that invasiveness can sometimes be reversed by biological control agents (e.g., prickly pear in Australia and Klamath weed in the American Pacific Northwest) suggests that invasiveness can appear simply once an organism is released from its natural enemies. Further, it has been observed that “a strong predictor of invasiveness . . . is whether the organism has been invasive . . . elsewhere” (Ewel et al. 1999).
Although such correlates may be statistically strong, they are typically biologically weak in predicting invasions, leading one reviewer of the field to assert, “serendipity is often an important element in successful invasions” (Gray, 1986) and another to lament,
“It could be that invasions . . . are intrinsically unpredictable” (Williamson, 1999).
But for some successful invasive species, it may well be that a series of events after colonization is more important than intrinsic colonizing ability. In fact, two phenomena commonly associated with successful invasions suggest that many species are not intrinsically invasive and that the right circumstances must transpire for them to become invasive. First, it often takes considerable time from the initial establishment of local populations of an exotic species to their aggressive spread. For example, Kowarik (1995) reviewed almost 200 invasive woody species with known dates of first cultivation in Brandenburg, Germany. The mean delay in invasion was 131 years for shrubs and 170 years for trees. Delays on the order of decades may occur for herbaceous invasive plants as well (Pyśek & Prach, 1993). If these species were simply preadapted, then we would expect evidence of invasiveness relatively quickly. But if they became invasive by evolutionary change, a lag time would be a prerequisite to spread. Second, a history of multiple introductions is a common feature for invasive species. For example, North America’s most successful invasive birds, the European Starling and the House Sparrow, both became invasive only after repeated introductions (Ehrlich et al., 1988). Multiple introductions might provide sufficient genetic diversity in a colonizing population which enables natural selection to create a subsequent adaptive response, invasiveness.
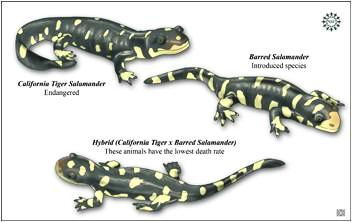
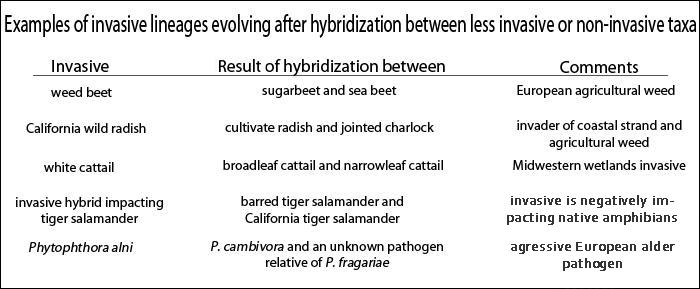
Blossey and Nötzold (1995) were the first to suggest that invasiveness could be the result of evolutionary change. Soon after, focusing mainly on plants, Ellstrand and Schierenbeck (2000) noted that hybridization may result in critical evolutionary changes that create an opportunity for increased invasiveness. Hybridization examples include Europe’s notorious weed beet, a natural hybrid between sugar beet and the wild sea beet that has wrought at least a billion dollars of damage to Europe’s sugar beet industry. While only a fraction of hybrid derivatives become invasives, such hybrid-derived organisms are emerging as important systems for the study of invasiveness (e.g., Biological Invasions. May 2009. Volume 11, Number 5). In less than a decade, the known number of invasive plants descended from hybrids increased 40%. The Table below gives a few well known examples.
It is now increasingly clear that hybridization is not the only evolutionary route to invasiveness. For example, a single gene mutation apparently is responsible for invasiveness of rose clover in California.
The recognition that invasiveness can evolve in species provides a tool for working to understand how some species become invasive. Research on such evolved invasiveness can pinpoint key changes that lead to invasiveness and therefore serve to inform how to prevent such future evolution.